Anju participated in the SCOPE conference (Summit for Clinical Ops Executives) last week in Orlando, Florida. For the first time, SCOPE held an attendee-voted Best of Show award for new technologies released within the last year. Anju submitted two entries for TA Scan – diversity data and site capacity calculator. Anju was voted as one of five Best of Show Award winners for innovative technology.
Diversity Data
Our clinical and commercial intelligence solution TA Scan with its integrated diversity data was one of these winners.
Business Problem
Clinical trials are highly complex and often bring significant challenges in patient recruitment and retention, owing in part to challenges with trial design and competition for patient and site resources. From a commercial perspective, the market is highly competitive and there is an ongoing need to demonstrate value and differentiation to relevant industry HCPs and thought leaders to protect market share.
Clinical and commercial teams rely on increasing amounts of data to provide them with historical and ongoing intelligence around competitive drugs and clinical trials, sites/PIs, patient-level data, and the HCP intelligence they need to engage as part of commercial efforts. However, clinical data is not standardized, and exists in hundreds of sources such as global registries, conferences, company websites, publications etc. Ensuring diversity of clinical trial participants is a key step in enabling health equity.
Although FDA has previously published guidance encouraging diversity in clinical trials, little progress has been made in improving the representation of racial/ethnic subgroups in trials of cancer therapies: in the trials supporting the 15 novel drug approvals for cancer indications in 2021, the majority of patients (72-95%) were White. Data strategies to ensure the inclusion of racially and ethnically diverse participants are becoming increasingly important. To date, however, there is a lack of robust and reliable data on race, ethnicity, and socio-economic characteristics on a global scale and without adequate data to identify relevant population groups, inequities remain unseen and unaddressed. The available diversity data is challenging and exists in different forms and structures.

Solution
TA Scan is a cloud-based clinical and commercial business intelligence tool that aggregates and connects global clinical trial data, presentation data, publication data, and many other data sources from the public domain into a single intuitive database. Our data represents an accurate, comprehensive view of global R&D activity, allowing users to make more informed decisions around their study designs, feasibility, site identification, and diversity and KOL identification and management strategies.
The TA Scan solution provides a quick and easy way to identify and evaluate data at any desired level of detail: clinical trial data, site information, publications, expert profiles, geographical mapping, competitive analysis, etc.
Despite the limitations in global diversity data, there are still a broad range of high-quality data which can be consolidated, standardized, and visualized to support streamlined diversity and site identification strategies. Our teams manage the complex data consolidation, ensuring global insights can be extracted to drive clinical trial equity and inclusion.
TA Scan now has fully integrated diversity data, allowing users to easily streamline their KOL, PI/site selection and diversity strategies, reviewing experience and diversity or socioeconomic data in the same visualization. Data can be filtered and visualized within the TA Scan application, exported from the tool for analysis, or alternatively delivered via API.
Innovative Technology
TA Scan technology collects and aggregates disparate and unstandardized sources at scale. Our patented TrialCloud technology allows us to semantically link all data types, which supports the extraction of hidden insights. Our processes and product are now enhanced with the addition of an extra data type to supplement TA Scan’s curated data into a single location. Diversity data is connected to graphs through geographies, following accurate processing of geographical units and providing proper granularity. In addition to complex data consolidation, our technology supports data visualizations to augment decision-making.
Implementing this new data process enhances the platform’s capabilities of including new data types such as other RWD.
Site Capacity Calculator
Anju also submitted another new TA Scan feature for the SCOPE Best of Show Awards: the Site Capacity Calculator.
Business Problem
Clinical trials are highly complex and often bring significant challenges in patient recruitment and retention, partly because of trial design problems and the competition for patient and site resources. From a commercial perspective, the market is highly competitive and there is an ongoing need to demonstrate value and differentiation to relevant industry HCPs and thought leaders to protect market share.
Clinical and commercial teams rely on increasing amounts of data to provide them with historical and ongoing intelligence around competitive drugs and clinical trials, sites/PIs, patient-level data, and the HCP intelligence they need to engage as part of commercial efforts. However, this data is notoriously challenging to find in the public domain. Clinical data is not standardized, and exists in hundreds of sources such as global registries, conferences, company websites, publications, etc. Sponsors and CROs have internal historical data and site lists: they tend to work with sites/PIs on their shortlist as the discovery of new sites/PIs with the capacity to recruit is challenging.
Identifying the ‘right’ sites for a particular study is essential to ensure the study’s success. This requires a data-driven approach to qualify sites for a particular study and limit unnecessary costs and delays in clinical trials. Site experience is key to study planning, but also understanding the competition for site resource and a site’s current capacity to recruit is critical. Even when sites may have experience and access to patients, are clinical teams considering a critical component – a site’s capacity to take on additional trials?
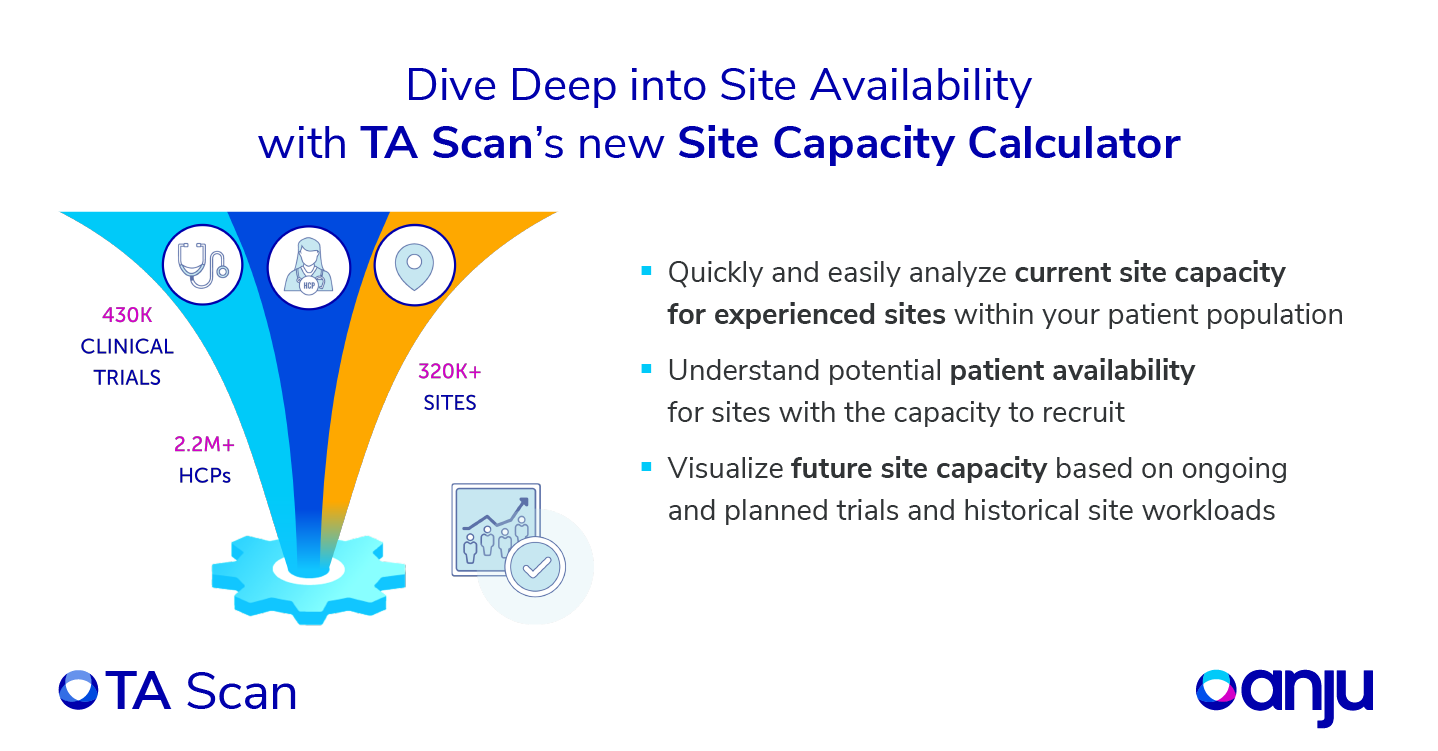
Solution
TA Scan is a cloud-based clinical and commercial business intelligence tool that aggregates and connects global clinical trial data, presentation data, publication data, and many other data sources from the public domain into a single intuitive database. Our data represents an accurate, comprehensive view of global R&D activity, allowing users to make more informed decisions around their study designs, feasibility, site identification, and diversity and KOL identification and management strategies.
The TA Scan solution provides a quick and easy way to identify and evaluate data at any desired level of detail: clinical trial data, site information, publications, expert profiles, geographical mapping, competitive analysis, etc.
TA Scan’s new site capacity calculator uses historical and competitive clinical trial data from multiple global clinical trial registries to forecast site performance for an upcoming study. To do so, TA Scan reviews site workload within specified search criteria (indication, specific patient population, phase, mode of action, …), over a defined period, so the overall activity today vs historical activity levels can easily be understood. With TA Scan Site Capacity Calculator, the user can explore the site capacity for a global list of sites or for a particular site in a period at a monthly level. The new site capacity calculator supports better, more informed site selection strategies to ultimately drive faster patient recruitment!
Innovative Technology
TA Scan technology collects and aggregates disparate and unstandardized sources at scale. Our patented TrialCloud technology allows us to semantically link all data types, which supports the extraction of hidden insights.
Our technology supports clinical trial merges and unmerges to assure accurate single data points and avoid duplicates in clinical trials which impacts site utilization and therefore historical capacity. Automated organization mapping is applied for correct trial – site relationships and TA Scan’s site inference algorithms can unlock undisclosed site data, providing a bigger picture of clinical trial landscape. This new feature further includes calculations for recruitment period estimation and assumes uniform enrolment on a study level by equal distribution of the enrolment target over sites. For all calculations, strict rules are followed, and data is only used when sufficient information is available (i.e., trial has two or more sites).
SCOPE Best of Show Awards
The inaugural Best of Show awards at SCOPE invited the more than 3,000 SCOPE attendees to vote on the best new products in the clinical trials and research space. Thirty-five companies exhibiting at the event entered nearly 40 new products launched since February 2022. The new products were on display in the SCOPE Exhibit Hall and event attendees were able to visit booths, see the new products in action, and ask questions before casting their votes for the top new products. The Best of Show competition is sponsored by Clinical Research News and ClinEco, the global clinical trials ecosystem and marketplace.
“Each year at SCOPE the creativity in the clinical research space is so inspiring,” said Clinical Research News editor, Allison Proffitt. “I’m thrilled that the Best of Show awards now formally recognizes those groups leading the charge with innovation.”
“What a wonderful launch year for the Best of Show Awards at SCOPE,” said Gregg Jewett, Director, Alliance Management at Genmab, and a ClinEco strategic advisor. “This first set of winners—chosen by the SCOPE community—represents solutions for some of the most challenging issues facing clinical research: increasing diversity in patient populations and developing platforms that put participant needs first without sacrificing data integrity. Congratulations to the 2023 winners.”
You can read more information on the SCOPE Best of Show awards and the other winners here.
Do you want to make data-driven decisions to accelerate your clinical trial timelines? Learn more about Anju’s Data Science Solutions and Data Science Services.
