ASCO 2023 is kicking off in Chicago, Illinois, and online. From June 2 to 6, oncology professionals from across the globe will drive conversations on the latest cancer research and share perspectives on the most sought-after innovations in oncology. This year, the global oncology community will focus on the theme ‘Partnering With Patients: The Cornerstone of Cancer Care and Research,’ taking a closer look at how interactions between clinicians and patients have changed over the years and how all people can receive the cancer care they need. Let’s dive into the highlights of ASCO23 that the Anju team has extracted for you.
ASCO23 at a Glance
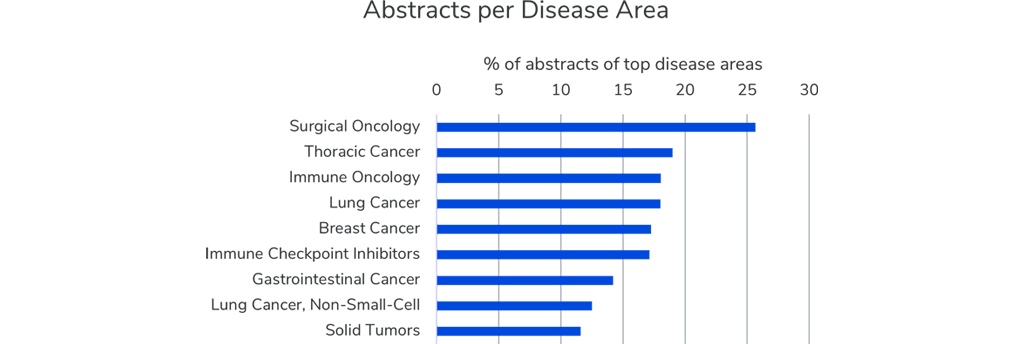
Around 6,000 abstracts will be presented at this year’s annual meeting. Breast Cancer is discussed in almost 1,000 presentations (17%). The number of abstracts for Lung Cancer (18%), Immune Oncology (18%) and Thoracic Cancer (19%) largely overlaps with just over 1,000 presentations each. Surgical Oncology takes the lead as the most discussed disease area and is referred to in over 25% of all presentations.
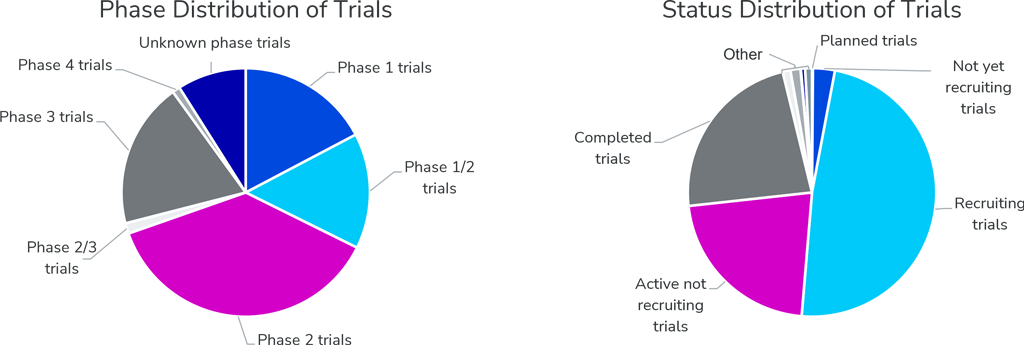
Like previous years, close to 30% of all abstracts are reporting updates and results of clinical trials. Most of the trials presented at ASCO are Phase 2 studies and over 70% of referenced trials are currently ongoing, showing that the latest insights in the oncology clinical landscape will be disclosed at this year’s meeting.
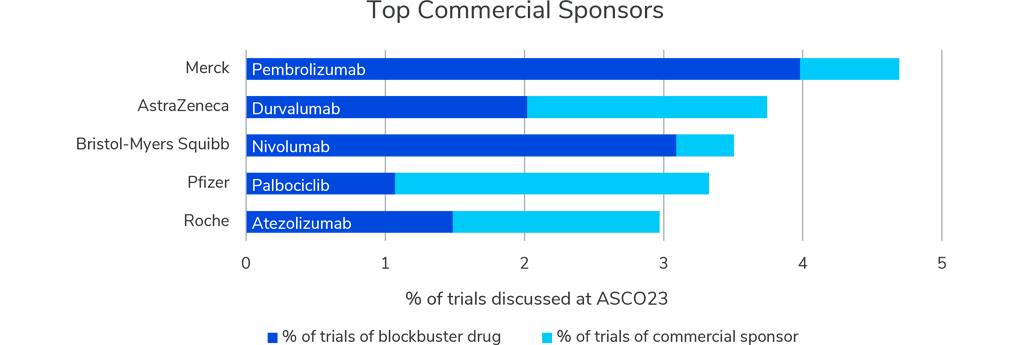
From a commercial point of view, over 350 commercial sponsors are involved with funding these trials, with Merck, AstraZeneca, Bristol-Myers Squibb, Pfizer, and Roche positioned as the top five commercial sponsors. The number of clinical trials presented this year for each of these sponsors’ blockbuster drugs is remarkable.
Novel Therapeutic Highlights
ASCO covers over 120 sessions featuring important and timely topics. One of the key focus areas at ASCO this year is novel immunotherapeutics and will give perspectives on checkpoints, bispecifics, and vaccines in development.
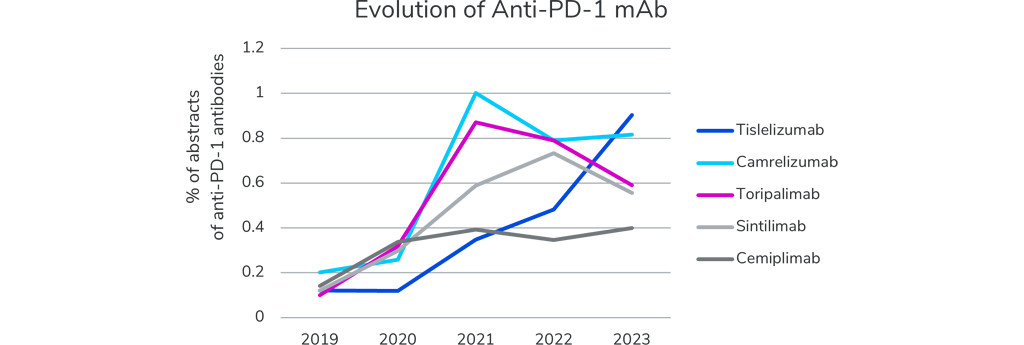
Overall, with over 1,100 drugs discussed, ASCO23 will highlight progress on a wide range of established and new therapeutic regimens. Like the previous five editions of the annual ASCO meeting, Pembrolizumab and Nivolumab (both anti-PD-1 antibodies) remain the #1 and #2 drug of interest, as shown by the large number of presentations that are allocated to research on these drugs.
At ASCO23, a few clinical reports are addressing how race and ethnicity impact response rates or the occurrence of adverse events of these checkpoint inhibitors. While the research of the best-selling drugs continues, five other anti-PD-1 monoclonal antibodies (Tislelizumab, Camrelizumab, Toripalimab, Sintilimab, and Cemiplimab) each passed the twenty presentations mark at ASCO 2023. All these antibodies have seen a clear increase in number of presentations, with a three- to seven-fold increase over the last five years. New anti-PD-L1 antibodies do not make a significant contribution to the conference. In general, for NSCLC, anti-PD-1 antibodies plus chemotherapy were shown to be superior to anti-PD-L1 antibodies plus chemotherapy. Yet, as monotherapy, both strategies appear to be similar. And anti-PD-L1 drugs were responsible for less severe adverse events (AEs), particularly, immune-related AEs.
Other rising antibodies and their targets to watch out for are anti-Lag3 antibodies (such as Relatlimab) and anti-CD47 antibodies (such as Magrolimab).
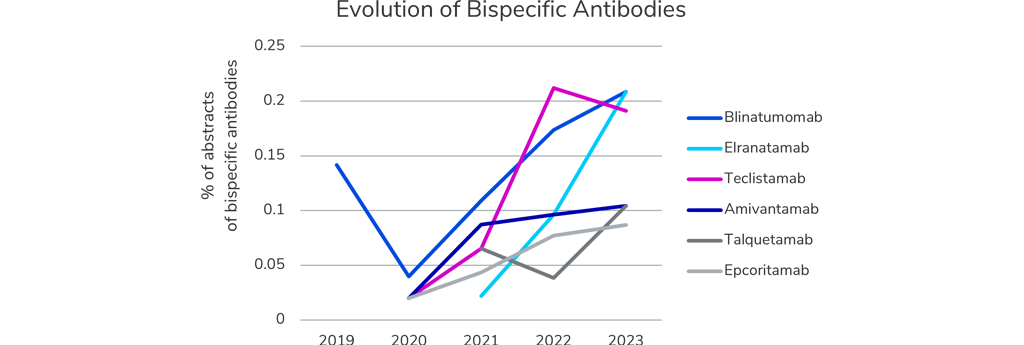
Bispecific antibodies are quite new on the block. Except for Blinatumomab, these bispecific antibodies are new to the oncology field and were not presented at the ASCO meeting five years ago. Yet, all of them are gaining rapid interest, as seen by the steep increase in number of abstracts over the last few years. Even though the targets between the antibodies differ, most are designed to bring T-cells and tumor cells together and activating the T-cells to kill the cancer cells.
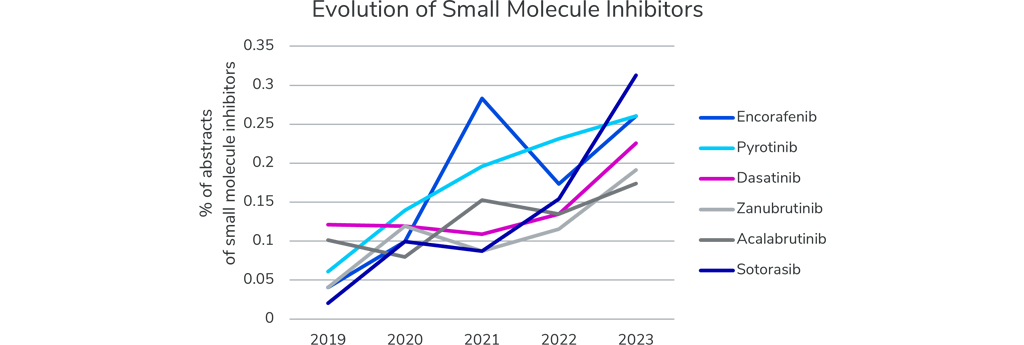
New small molecule inhibitors are also making their way into the field as novel treatment options for cancer patients. The graph below highlights six novel small molecule inhibitors that are clearly on the rise, based on their increasing number of abstracts at the ASCO meetings.
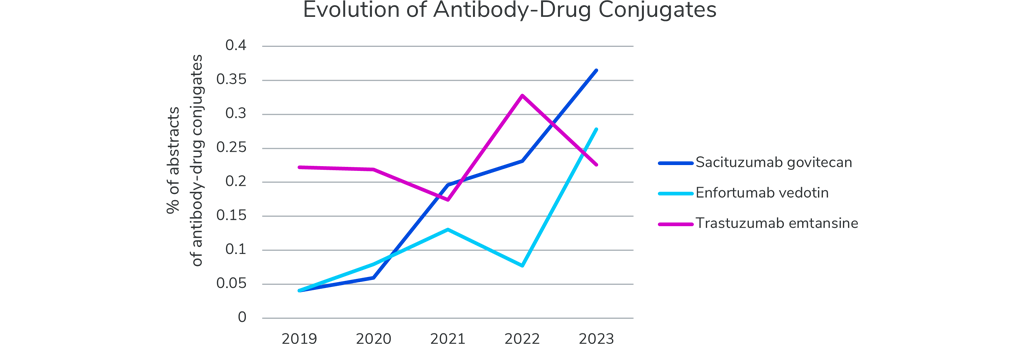
Last year, we were excited about emerging antibody-drug conjugates, which are making a return this year. Just like last year, antibody-drug conjugates targeting Her2 (such as Trastuzumab emtansine) and Trop2 (such as Sacituzumab govitecan) take the lead in the antibody-drug conjugate market, with 50 and 24 presentations respectively. Antibody-drug conjugates targeting Nectin-4 (such as Enfortumab vedotin) also see a steep increase in number of presentations at the ASCO meeting. There are at least fifteen different antibody-drug combinations being investigated and discussed at ASCO23. We are looking forward to the promising results and the advancement of successful antibody-drug combinations.
Take-Home Message
The ASCO 2023 Annual Meeting will once again unveil enticing clinical data from pivotal research across malignancies. We clearly see drug development move forward year over year. With the focus on enhancing innovative therapies to meet the needs of patients, the industry requires effective strategies to improve trial diversity among underserved populations. Only then can we truly gauge how these treatments may provide real-world benefits to those most impacted by certain cancers.
The innovative mindset at ASCO combined with the exploration of clinician and patient interaction promises to drive progress and find tangible ways the industry can act to keep the patient front and center in drug development.
This report only gives a sneak peak of what to expect at this year’s meeting. ASCO23 further features sessions on personalized immunotherapy, CAR T-related toxicities, the next generation antibody-drug conjugates, and treatment options in gastrointestinal cancers beyond checkpoint inhibitors. Moreover, the most up-to-date results will only be revealed once ASCO kicks off this weekend and industry-relevant ASCO late-breaking abstracts are released on a rolling basis. We eagerly look forward to all study results that could once again shift practice in cancer care.
Advance Your Oncology Trials with Anju’s Innovative Technology Solutions
Anju is committed to helping our clients treat and, one day, cure cancer. Are you attending the 2023 ASCO Annual Meeting? Is your team in need of cost-efficient and adaptable Data Science and eClinical solutions to optimize and accelerate the execution and management of your (decentralized) complex oncology clinical trials?
Book a meeting with our CEO Larry Birch, our VP of Business Development George Naxera, or our Director of Business Development David Kirschenbaum! Anju is your trusted technology solutions partner from concept to cure.

TA Scan: A 360° Panoramic View of Your Therapeutic Landscape
TA Scan is a comprehensive, web-based clinical and commercial business intelligence solution. It aggregates and analyzes clinically relevant public data to facilitate and accelerate data-driven decision-making for all aspects of clinical study planning and implementation through data insights and analytics.
The TA Scan team also provides a broad range of data consolidation, integration, and delivery services. We can tailor custom solutions to your business requirements. Our extensive data and data linking experience can rapidly connect multiple data sources, driving clinical and commercial team efficiency.
