April 11 marks World Parkinson’s Day! A time to shine a light on Parkinson’s disease and empower yourself with data on the current clinical research landscape and the latest advancements.
 Parkinson’s disease is the second most common neurodegenerative disorder [1] affecting over 10 million people around the world [2]. Although the disease has no cure, the development of levodopa in the 1960s represents one of the most important breakthroughs in the history of medicine [3]. Parkinson’s has united global healthcare professionals and prominent pharma companies with an aim to provide the best treatment regimen for this disease.
Parkinson’s disease is the second most common neurodegenerative disorder [1] affecting over 10 million people around the world [2]. Although the disease has no cure, the development of levodopa in the 1960s represents one of the most important breakthroughs in the history of medicine [3]. Parkinson’s has united global healthcare professionals and prominent pharma companies with an aim to provide the best treatment regimen for this disease.
Hope through research
3,321 trials and 1,759 sponsors have brought together 6,331 global investigators. The most active regions (in number of trials) are Europe (1,273), North America (1,094), and Japan (305). With 698 currently ongoing trials, of which 458 started during the COVID-19 pandemic, it is clear that this disease area has not lost any awareness.
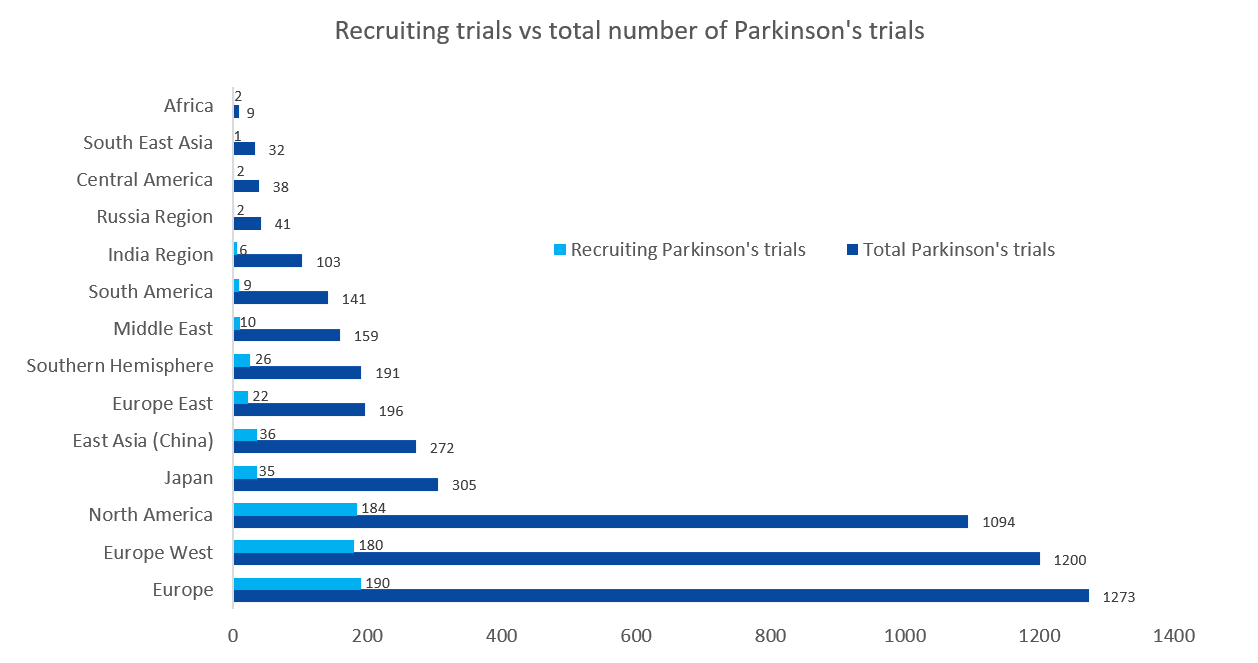
Access TA Scan to quickly identify 1,950 investigators, their collaborators, and 1,623 clinical study sites associated with these ongoing trials. Most of these trials (82%) are academically sponsored and conducted in academic hospitals and medical centers. However, Abbvie, Boston Scientific Corporation, and InSightec are the leading commercial sponsors in this domain.
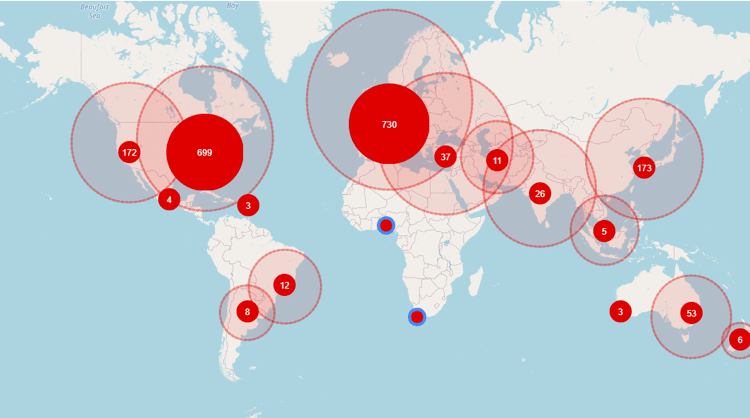
Global distribution of investigators for ongoing trials in Parkinson’s Disease
Drugs under the lens
Currently, Parkinson’s has 188 registered drugs (registered either in the US or EU). Levodopa, carbidopa, and a combination of levodopa + carbidopa are the drugs with the greatest number of ongoing trials. However, pharmaceutical companies continue their efforts to deliver new treatments by the end of 2024 [4].
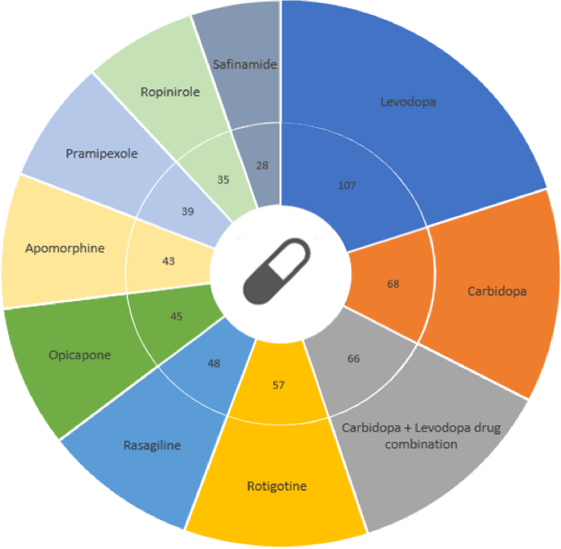
Top 10 Parkinson’s drugs: trial count
Most of the “new drugs” are in their Phase 2 development, such as psilocybine [NCT04932434], 4-chlorokynurenine [NCT04147949], traneurocin [NCT03538522], ath1017 [NCT04831281], PG203 [2020-006004-16] and prasinezumab [NCT04777331, NCT03100149]. You can access these Gantt charts in TA Scan to visualize the trial timelines, hover over to get key trial information, or click on a trial ID to obtain more details through a trial drill-down.
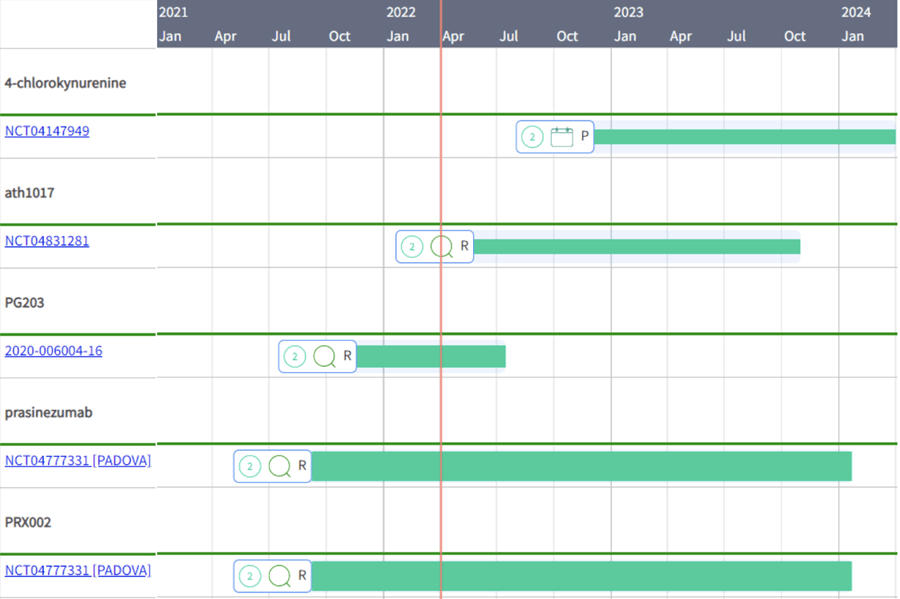
Trial timeline for “new drugs” in Parkinson’s Disease
Stay informed with TA Scan
Are you interested in knowing more about Parkinson’s Disease or any of our other 525 therapeutic areas? Our clinical intelligence solution TA Scan allows you to extract relevant information and perform powerful analytics to get faster, data-driven insights for your business objectives ranging from Clinical Operations to Medical Affairs.
If you have any questions regarding TA Scan, please contact us or schedule a demo.
