Multiple Sclerosis (MS) is one of the most common diseases of the central nervous system affecting 2.8 million people around the world (Cotsapas et al., 2018). Today, on World Multiple Sclerosis Day, let’s discuss the current clinical research landscape, latest advancements, and more.

Multiple Sclerosis (MS) is a potentially progressive, autoimmune-mediated neurodegenerative disease of the central nervous system characterized by inflammatory demyelination with axonal transection. The main agents responsible for this disease include exogenous, environmental, and genetic factors. Typically present in young adults, it can lead to physical disability, cognitive impairment, and decreased quality of life, with no known cure. Emerging disease-modifying therapies aim to control symptoms, with increasingly sophisticated immune function modulation (Cotsapas et al., 2018; Kamińska et al., 2017; McGinley et al., 2021).
Hope through research
With 5,355 research sites, 1,327 sponsors, 2,844 trials and 6,334 investigators, this disease did not lose any awareness. The most active regions (in number of trials) are North America (2,635), Europe (2,430) and Middle East (502). At this moment, there are 546 ongoing trials, all of them starting from last year.
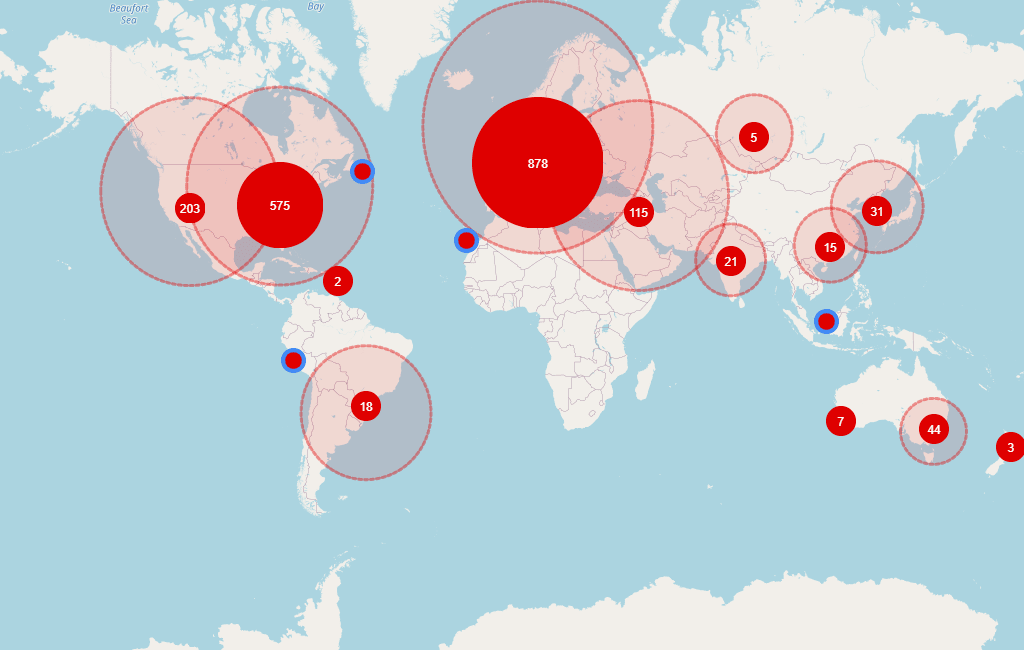 Global distribution of investigators involved in ongoing trials in MS
Global distribution of investigators involved in ongoing trials in MS
Most of these ongoing trials are academically sponsored. However, Roche SA, Novartis SA, and Biogen Inc. are the leading commercial sponsors in this domain.
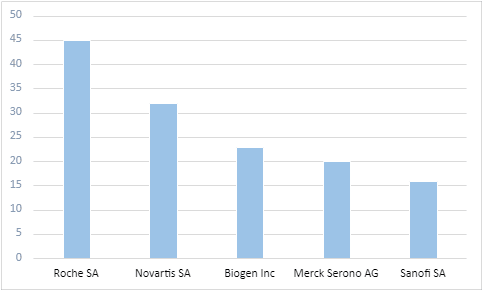
Top commercial sponsors from ongoing trials in MS
Collaboration: a substantial key to a successful cure
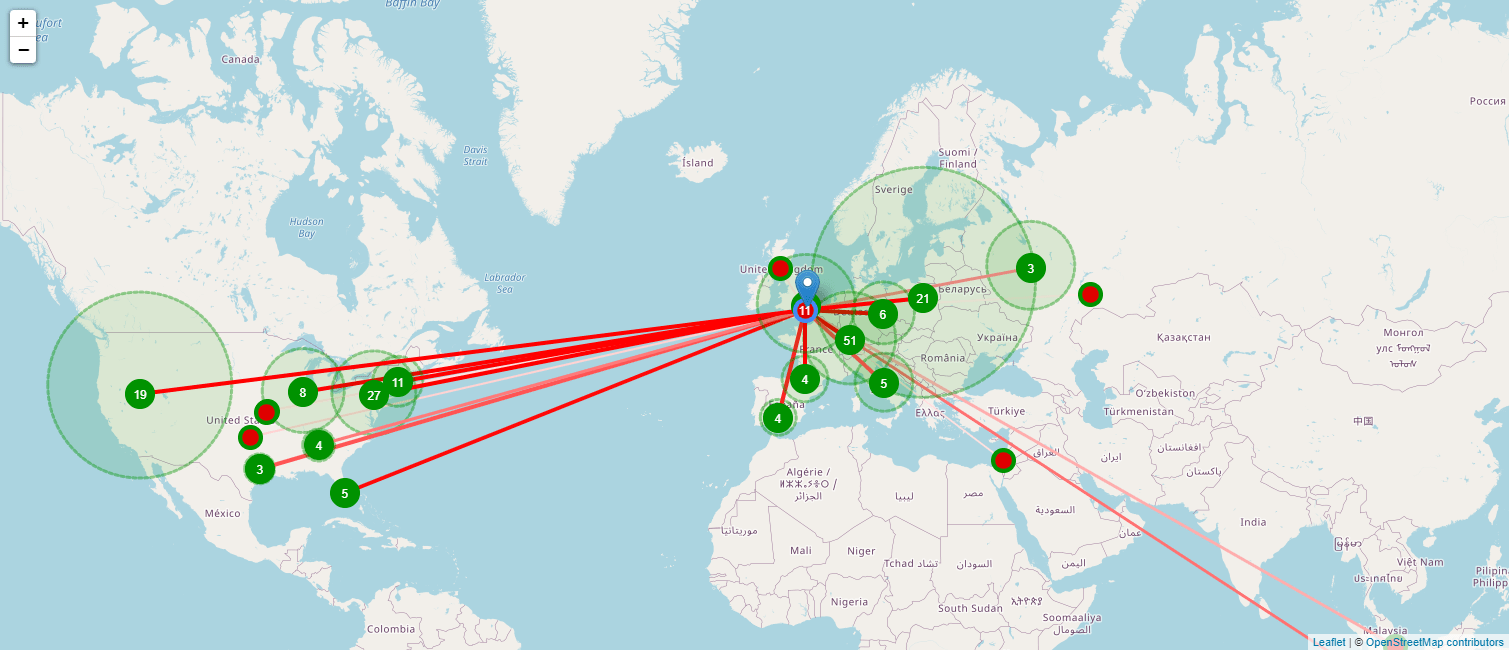
Owing to the complicated nature of this disease, finding promising treatments for MS requires close collaborations between various experts around the world. With TA Scan you can identify a global expert in any therapeutic field, and also identify and map other experts they have worked with. The map below shows you such a network, with the color of the line indicating how often they have collaborated: the darker the line is, the stronger the collaboration.
So far 168 drugs are being used to treat MS, including ocrelizumab: the drug with the greatest number of ongoing trials. Ocrelizumab is a monoclonal antibody, designed to target a particular marker (CD20) on the surface of B cells, which is thought to be involved when the immune system attacks the myelin around nerve cells, leading to the target B cells being destroyed. However, pharmaceutical companies continue their efforts to push deliver new treatments in the upcoming years.
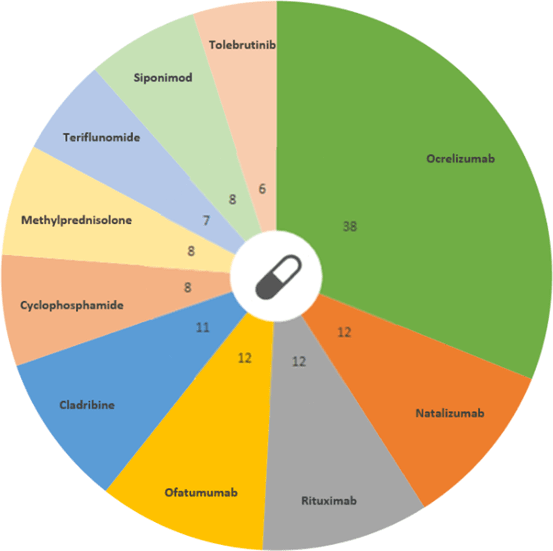
Drug distribution from ongoing trials in MS
From the 546 currently ongoing clinical trials, 62 are in phase 2 and 88 in phase 3. With TA Scan you can compile your primary site list for the next trial phase. TA Scan will help identify sites with experience in phase 3, but which are currently not running any trial, and have the capacity to enroll the patients needed.
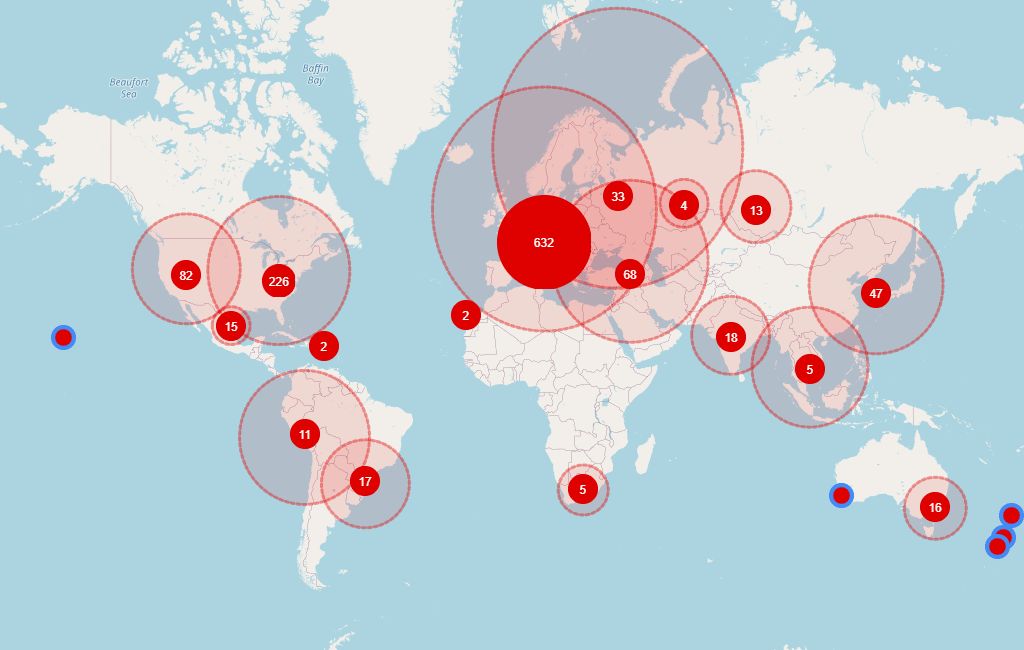
Disclosed/inferred sites with phase 3 experience not running any trial
Stay informed with TA Scan
Are you interested in knowing more about Multiple Sclerosis or any of our other 525 therapeutic areas? Our clinical intelligence solution TA Scan allows you to extract relevant information and perform powerful analytics to get faster, data-driven insights for your business objectives ranging from Clinical Operations to Medical Affairs.
If you have any questions regarding TA Scan, please contact us or schedule a demo.
References
Cotsapas, C., Mitrovic, M., & Hafler, D. (2018). Multiple sclerosis. Handbook of Clinical Neurology, 148, 723–730.
Kamińska, J., Koper, O. M., Piechal, K., & Kemona, H. (2017). Multiple sclerosis – etiology and diagnostic potential. Postepy Higieny i Medycyny Doswiadczalnej (Online), 71(0), 551–563.
McGinley, M. P., Goldschmidt, C. H., & Rae-Grant, A. D. (2021). Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA, 325(8), 765–779.
